Our content continues to evolve; please check back often for updates.
Update for Week of Nov 23
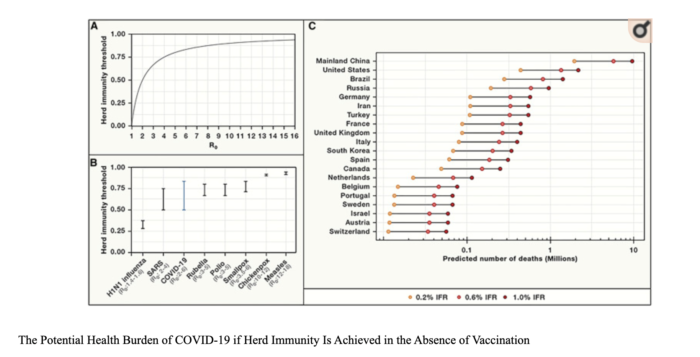
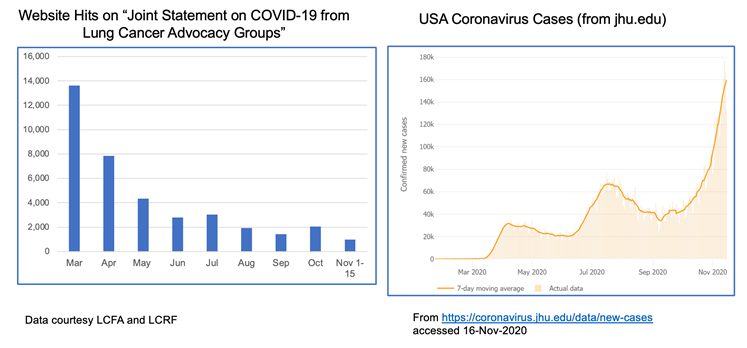
We are at a critical moment in the ongoing COVID-19 pandemic. New cases are rapidly escalating throughout the country, and we are positioned to see explosive growth as people travel and gather to celebrate the Thanksgiving holiday with loved ones. While our understanding of how to treat COVID-19 has grown significantly since the disease first burst onto the scene, deaths continue to mount, with the US now seeing the most daily deaths since May.
The realities of the current situation are compounded by our collective national fatigue and desire to return to some sense of normalcy. When we look at website hits for these joint statements over time, we see a lot of activity in the spring when COVID-19 was “new,” but those numbers have dropped off substantially through the summer and fall. This stands in stark contrast to the growth of cases through subsequent waves of infection.
The take home message is that we must not let our guard down! Please continue to wear a mask, watch your distance and wash your hands. Our collective actions over the next few weeks CAN make a difference in helping curb the recent surge. We also recognize the importance of balance, particularly for patients with cancer who fear they may not have another Thanksgiving or Christmas. For practical guidance on how to navigate your holidays safely, please refer to this helpful discussion.
Despite the current situation, there is reason for hope. We can now see the light at the end of the tunnel with the recent announcements that both Moderna and Pfizer/BioNTech have developed highly effective COVID-19 vaccines, with others in the pipeline. You can find a comprehensive overview of how vaccine trials work and current vaccine efforts underway here.
Additionally, monoclonal antibody therapies continue to make progress. Eli Lilly recently received Emergency Use Authorization from the FDA for its antibody therapy in recently diagnosed, high-risk patients. Regeneron also received a lot of press when its antibody therapy was used to treat President Trump.
VACCINE FAQS
The development of a new class of mRNA-based vaccines has raised many questions, particularly among the lung cancer community. We have been collecting these questions and will do our best to address them here.
- How do mRNA vaccines work?
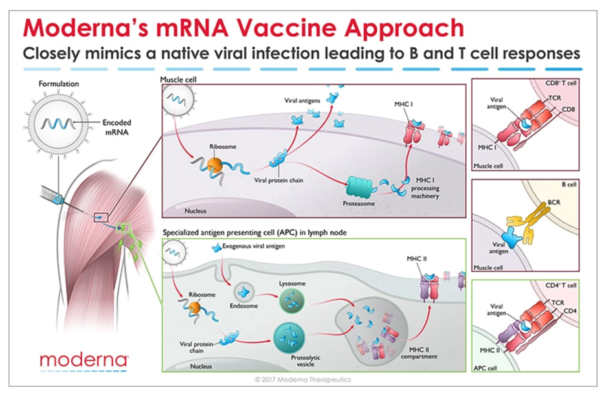
Messenger RNA (mRNA) is the recipe for making a protein. The mRNA gets injected into the body and is taken up by cells that “read the recipe” for making the SARS-CoV-2 spike protein. This is the protein normally expressed as a “crown” on the virus particle and is the part of the virus that binds to the receptor found on cells in the lungs and in other tissues throughout the body. Once these cells take up the mRNA and make the spike protein, they can display pieces of spike on their cell surface to signal the immune cells to become activated. B cells are a type of immune cells that make antibodies that can block virus binding. CD4 T cells support B cells to make antibodies while CD8 T cells can kill virus-infected cells. This is illustrated in the figure below for Moderna’s vaccine (though Pfizer/BioNTech’s vaccine works in the same manner).
- How do we know these vaccines are safe?
All new drugs and vaccines go through extensive testing as part of the clinical trials process. (summarized in the NYTimes link above). Both the Moderna and Pfizer/BioNTech vaccines are currently in Phase 3 clinical trials, reporting nearly 95% efficacy and no significant safety issues. It is important to note that these trials have been conducted in thousands of patients. However, no significant safety issues does not mean the vaccines don’t come with some unpleasant side effects which are short-lived. Those effects should not be a reason to avoid the vaccine. Educating healthcare providers on the mRNA technology and ensuring them that the vaccines are safe will be key to a successful rollout.
- When will the vaccines be available? Will patients with lung cancer be prioritized?
Based on the safety and efficacy profiles of both vaccines, it is expected that people will start receiving them before the end of the year, perhaps as soon as December 12 in the US. Many national experts are developing guidance for vaccine distribution, with the National Academies issuing a framework that would see healthcare workers, frontline workers and those in high-risk categories being eligible to be vaccinated first. Given that several studies have now reported high mortality rates in patients with lung cancer who contract COVID-19 , it is widely expected that lung cancer patients would be among those first eligible to receive the vaccine in the early stages of rollout.
- Should I take the first vaccine available or wait for a later generation one?
As stated earlier, both the Moderna and Pfizer/BioNTech vaccines are highly effective with a strong safety profile. There have been fears among many that the rush to produce a vaccine would result in compromised safety or efficacy but adherence to standards established by the FDA and other agencies assures us that these vaccines are safe.
It is important to note that before mRNA vaccines were developed in the fight against COVID-19, they were being developed to help combat cancer. Both Moderna and BioNTech (the company that partnered with Pfizer on its COVID-19 vaccine) have been developing mRNA vaccine technology for some time in the hopes of using this approach to treat various forms of cancer as well as other infectious diseases.
Given the unique threat that COVID-19 presents to the lung cancer community, we strongly encourage you to have a discussion with your doctor about getting the vaccine as soon as it is available to you. As for choosing between these two specific vaccines, the technology is essentially identical. Both require two shots over the course of a few weeks. The differences come down to logistical challenges of ensuring facilities have proper freezers for maintaining the vaccines at the appropriate subzero temperatures.
Other vaccine candidates are in development that use different technology platforms. It remains possible that some future vaccines may require only a single dose (such as Janssen’s vaccine) or be administered differently (intranasal vs injection).
Until those vaccines gain approval, the current decision will be based on availability of the two mRNA-based vaccines.
It is worth noting that a multi-institutional, NCI-funded grant has been awarded to study antibody responses to SARS-CoV-2 infection in lung cancer patients as compared to healthy people. This effort will try to answer why lung cancer patients seem to have worse outcomes from COVID-19 and will study responses in patients receiving a vaccine compared to those who do not.
UNANSWERED QUESTIONS
Several questions remain about the new mRNA vaccines:
- Can these vaccines completely prevent infection, or will they just prevent symptoms from developing?
- Can people who receive the vaccine still transmit the virus to others?
- How long will any resulting immunity last? Previous results from these types of vaccines in other settings suggest that protection may wane after a year.
More data is needed before we can answer these questions.
FINAL TAKEAWAY
There is no escaping the seriousness of our current national crisis – COVID-19 cases are increasing everywhere and so we must do what we can to protect ourselves and our loved ones a little while longer.
However, hope is on the horizon. We can face 2021 knowing that, through the power of science, this pandemic will eventually come to an end.
HAPPY HOLIDAYS AND PLEASE STAY SAFE!
Resources and websites
- IASLC’s Guide to COVID-19 and Lung Cancer
- The National Cancer Institute website for COVID-19 and emergency preparedness COVID-19: What People with Cancer Should Know
- Updates from the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC)
- Johns Hopkins COVID-19 Resource Center
- Interactive map of US COVID-19 cases by state
- COVID-19 in patients with cancer: managing a pandemic within a pandemic
- You can find information specific to your state or city or town on your health department’s website.
- American Medical Association resources for healthcare providers.
Update for Week of October 19, 2020
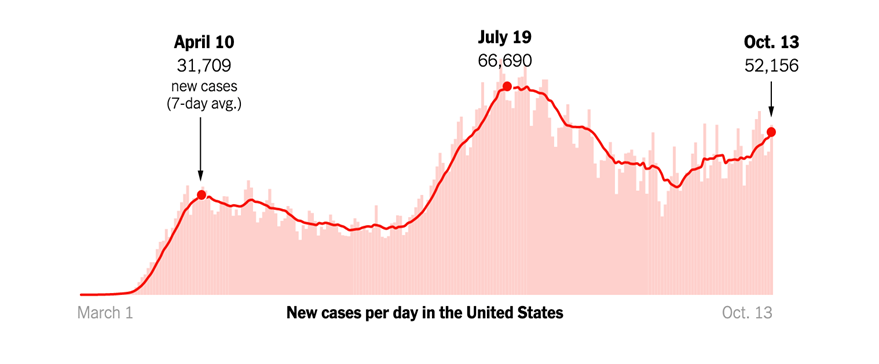
The daily news reports are a stark reminder that the COVID-19 pandemic is far from over. Consistent with experts’ fears for the fall, new cases are on the rise across the US and in Europe.
Caught in the grips of this unprecedented public health crisis for almost all of 2020, Americans are growing fatigued and restless. The lockdowns in the spring and the extended period of social distancing needed to keep the virus at bay are negatively impacting people’s mental health. For many, it is the lack of touch, a simple hug, that we miss the most.
And this is the time of year when we start looking to Thanksgiving to reunite with family and loved ones, a time often celebrated with large gatherings, extended celebrations and warm embraces. But, at a time when cases are once again surging across the country, each of these activities presents a serious risk for virus transmission. This risk comes at even greater cost for the lung cancer community given the increased likelihood of severe disease and heightened mortality for lung cancer patients who contract COVID-19.
Recently, several health experts have weighed in on how best to approach the holiday to ensure maximal safety. Dr. Anthony Fauci, the nation’s leading infectious disease expert, has suggested Americans need to strongly weigh the risk-benefit of having Thanksgiving gatherings. In places or states with a high number of new cases, some experts even advise canceling (or at least postponing) this year’s celebration. You can check each state’s COVID-19 new case activity here.
While we all feel the need to be close to our loved ones at this time of year especially, we want to urge all of you to do your homework and take appropriate precautions to protect yourselves and those around you. You can use a risk calculator to decide the level of risk. To assist with your planning, the CDC also provides a list of Thanksgiving activities at different risk levels. The table below offers example activities at different risk levels for virus spread.
| Low Risk | Moderate Risk | Higher Risk |
| · Having a small dinner with people who live in the same household
· Having a virtual dinner with your loved ones and make it fun by sharing recipes · Preparing special family recipes and delivering them in a safe and contact-free fashion |
Having a small outdoor dinner with family and friends who live in your community while maintaining social distancing | · Attending indoor gatherings with people from outside your household
· Attending large indoor celebrations with singing or chanting |
|
Watching a sporting event in a virtual get-together |
Attending a small outdoors sports event where public health precautions are maintained | Attending a crowded sports event, even if it’s outdoors |
| Watching all Thanksgiving events (parade, sports) from home | · Attending a pumpkin patch or orchard where people are following public health precautions
· Having a small group outdoor, open-air parade with social distancing
|
Attending or participating in crowded parades |
| Shopping online after Thanksgiving | Going shopping in crowded stores around Thanksgiving holiday |
We realize that celebrating the holidays is an important part of our tradition. We, therefore, suggest that you identify an inner circle of family and friends (your social distancing crew) who will be taking precautions with you during the holidays so you can celebrate safely! The holidays can be stressful, and with the pandemic adding a new layer of stress, do not forget to take care of your mental health.
How can you vote safely during the pandemic?
Election day is coming, and it’s important to make your voice heard. If you’re concerned about how to vote safely during a pandemic, Consumer Reports offers a Guide to Voting During a Pandemic that covers several different approaches to voting. The CDC has also issued special COVID-19 safety recommendations for voters. Many of their suggestions are familiar by now; however, the CDC also discusses additional precautions specifically for in-person voting. Some examples:
- Avoid delays by verifying your voter info and having any necessary registration forms ready.
- Bring your own black pen (or stylus, if used in your precinct).
- Review a sample ballot in advance so you can vote and depart quickly.
- Use early voting, if available in your jurisdiction.
- Vote at off-peak times, such as mid-morning.
- If driving to the polls and your schedule allows, monitor the voter line from your car and join it when it’s shorter.
Resources and websites
- IASLC’s Guide to COVID-19 and Lung Cancer
- The National Cancer Institute has a special website for COVID-19 and emergency preparedness. COVID-19: What People with Cancer Should Know
- We are following updates provided by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC)
- Johns Hopkins COVID-19 Resource Center
- Interactive map of US COVID-19 cases by state
- COVID-19 in patients with cancer: managing a pandemic within a pandemic
- You can find information specific to your state or city or town on your health department’s website.
- American Medical Association resources for healthcare providers.
Update for Week of September 21, 2020
As of September 18, 2020, the US has had 6.7 million cases of COVID-19, with just over 198,000 deaths. The Midwest is leading new cases, with 8 cities in Wisconsin appearing on The New York Times list of the 20 metro areas with fastest-growing cases.
With the run-up to the US Presidential election now less than two months away, recent weeks have seen a growing national dialog on the potential availability of a SARS-CoV-2 vaccine. In this week’s update, we want to review some basic concepts on vaccines, the clinical trials process for ensuring vaccine safety and provide an update on the current status of the various vaccine candidates currently under development.
What is a vaccine? How long do vaccines last?
In the most basic terms, a vaccine is a substance that can stimulate the body’s immune response to provide protection against diseases caused by different viruses and bacteria. Some vaccines provide potentially life-long protection (measles) while others provide long-term protection but still require periodic “booster” shots (tetanus being a classic example). Still others require annual vaccination because of the nature of the virus – influenza virus (that causes “flu”) undergoes changes from year to year and so the formulation for the vaccine changes each year to accommodate these changes and offer the best protection possible.
(PSA: don’t forget to get your flu shot this year!)
How are vaccines tested?
Everyone feels a great sense of urgency to develop a vaccine for SARS-CoV-2 so we can think about returning to some degree of “normalcy” in our daily lives. A concerted global effort is currently underway not only to develop a safe and effective vaccine but to develop other treatments as well (including so called monoclonal antibodies as well as novel antiviral treatments). In the US, the administration has developed what it refers to as “Operation Warp Speed” to try to accelerate vaccine development.
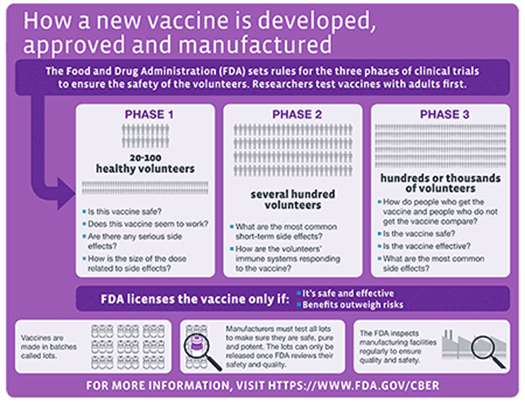
Without getting into a political debate, we want to offer a brief overview of what goes into getting a vaccine approved. Specifically, once a candidate vaccine is identified, its safety and efficacy (how well it works) must be validated through a rigorous clinical trials process as shown in the schematic below:
For a great overview of how vaccines are developed, the different types of vaccines, how they are tested and the status of current efforts to develop a SARS-CoV-2 vaccine, we refer you to an excellent resource put together by The New York Times.
Vaccine Safety
Historically, the United States Food and Drug Administration’s Center for Biologics Evaluation and Research (CBER) has been responsible for regulating vaccines in the US. Recently, the scientific integrity of both the FDA and the Centers for Disease Control and Prevention (CDC) have come into question over fears that they may be rushing vaccine development in the interest of political expediency. Because of this concern, many of the pharmaceutical companies at the forefront of the effort to develop a SARS-CoV-2 vaccine signed an unprecedented pledge affirming their commitment to vaccine safety.
Politics aside, the scientific community must ensure any potential vaccine is both safe AND effective before it is approved and administered to the public. Past experience with the development of SARS and MERS (Middle-Eastern Respiratory Syndrome) vaccines has taught us that coronavirus vaccines need thorough testing. A recent incident that occurred during the Phase 3 clinical trial of AstraZeneca’s vaccine candidate highlights why vaccine safety is paramount. The initial lack of details about the nature of the incident raised concerns about lack of transparency by the drug companies developing these vaccines. In response to mounting pressure, several of the leading contenders have made their protocols public.
Hope on the Horizon
Despite the challenges associated with developing an effective vaccine against SARS-CoV-2, there are several reasons to be hopeful:
- The science is advancing at a historic and unprecedented pace. Previously, the fastest vaccine ever made (against mumps) took four years to develop.
- We have access to novel vaccine development platforms and also experience with coronavirus vaccine development with SARS and MERS. Scientists are building on this pool of available knowledge to develop a vaccine against SARS-CoV-2.
- We have gone from first identifying a novel virus (SARS-CoV-2) as the cause of COVID-19 (Dec 2019) to having the sequence of the viral genome (Jan 2020) and the pursuit of multiple, compelling vaccine efforts within the span of only six months.
Resources and websites
- IASLC’s Guide to COVID-19 and Lung Cancer
- The National Cancer Institute has a special website for COVID-19 and emergency preparedness. COVID-19: What People with Cancer Should Know
- We are following updates provided by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC)
- Johns Hopkins COVID-19 Resource Center
- Interactive map of US COVID-19 cases by state
- The One-Two Punch: Cancer And COVID-19 (an important perspective for cancer patients)
- You can find information specific to your state or city or town on your health department’s website.
- American Medical Association resources for healthcare providers.
Update for Week of September 7, 2020
We hope that all of you had a peaceful Labor Day holiday. This week marks the six-month anniversary of when the World Health Organization declared COVID-19 a global pandemic (March 11). As of September 7, 2020, cases in the US have surpassed the 6 million mark, with over 186,000 deaths.
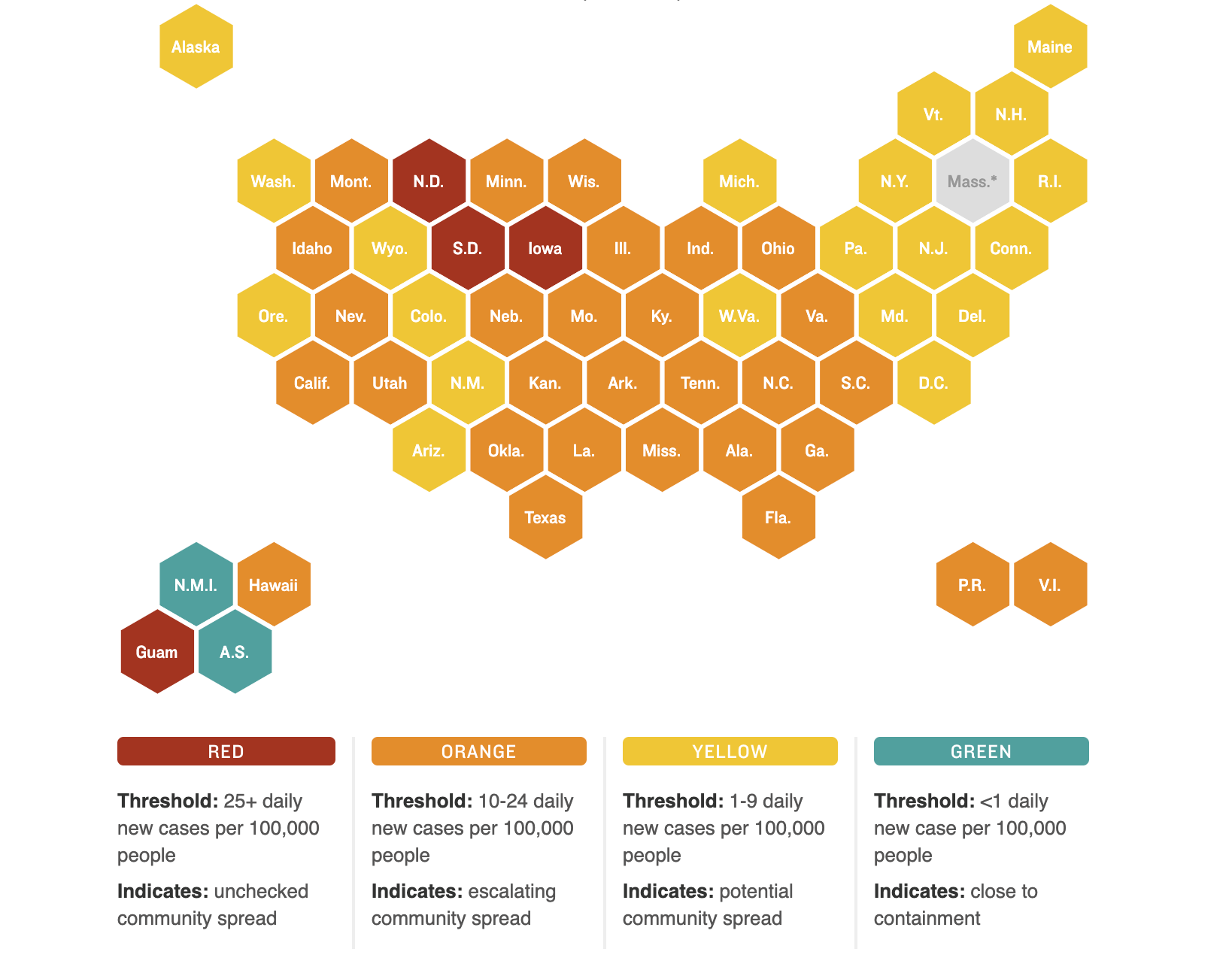
Nationally, new cases appear to be on a decline but pockets of high COVID activity remain. The figure below shows which states have the most new daily cases and the relative degree of community spread versus containment of the virus:
PSA: Get your flu shots!
With the arrival of September, we are strongly recommending that all eligible patients and caregivers get their annual flu shot this year! Public health experts are particularly concerned about the potential for patients to get infected with both influenza and SARS-CoV-2 this winter. Additionally, since the symptoms for these two viruses are similar, many patients experiencing flu-like symptoms may flood already overtaxed healthcare systems. Many doctors’ offices and pharmacies already have flu shots available. It’s also important to remember that it takes approximately two weeks from receiving the shot to have adequate protection. So please make a plan to get your shot as soon as possible.
Some patients, particularly those on checkpoint inhibitors, may be concerned about whether they can take the flu shot – we always recommend asking your doctor but previous studies suggest that it is safe for patients.
We want to hear from you!
We are interested in knowing what topics we should cover in future updates. Please share your thoughts with us by taking this short (1-2 minute) anonymous survey.
https://www.surveymonkey.com/r/LungAdvocacy_COVID19_needs
Resources and websites
- IASLC’s Guide to COVID-19 and Lung Cancer
- The National Cancer Institute has a special website for COVID-19 and emergency preparedness. COVID-19: What People with Cancer Should Know
- We are following updates provided by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC)
- Johns Hopkins COVID-19 Resource Center
- Interactive map of US COVID-19 cases by state
- The One-Two Punch: Cancer And COVID-19 (an important perspective for cancer patients)
- You can find information specific to your state or city or town on your health department’s website.
- American Medical Association resources for healthcare providers.
Update for Week of August 10, 2020
As of August 9, 2020, we are approaching 20 million cases of COVID-19 worldwide, with almost 5 million cases and 160,000 deaths in the US alone. In this week’s update, we want to shift our attention to another looming healthcare crisis resulting from the pandemic, namely a significant decline in new cancer diagnoses. Given the importance of maintaining appointment schedules, we will also present questions that you may want to ask your healthcare provider in advance of visits to the doctor. Finally, we will highlight ongoing advances in lung cancer research, because cancer doesn’t stop and neither do we.
What is the impact of COVID-19 on new cancer diagnoses?
In the early days of the pandemic here in the US, many stakeholders conducted various modeling simulations to look at the short-term and long-term impacts of the pandemic, particularly related to people continuing to get their recommended cancer screenings (mammograms, colonoscopies). These studies highlighted a looming crisis, predicting a rapid decline in the number of new cancer diagnoses. Dr. Ned Sharpless, Director of the National Cancer Institute, highlighted some of this data in a recent presentation at the AACR COVID-19 and Cancer Conference and in an editorial for Science.
This past week, a new study showed an alarming overall drop (46%) in new cancer diagnoses across six different tumor types, including lung cancer, for the period from March 1 to April 18, 2020:
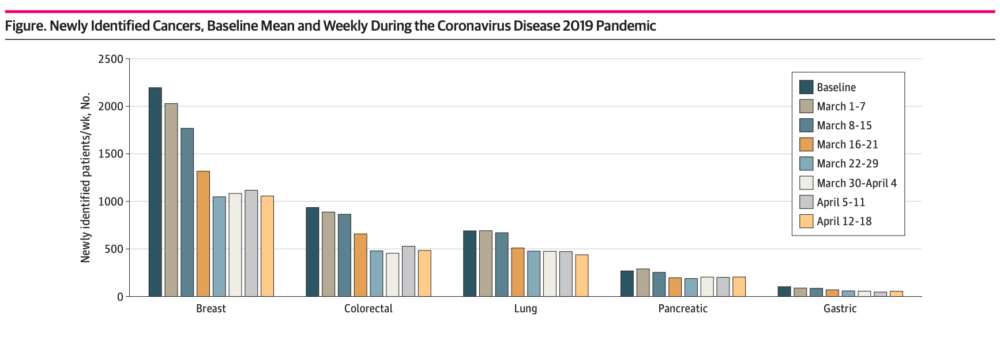
Additional reports from the across the country indicate an even higher drop in new cancer diagnoses. The COVID and Cancer Research Network reported a decline of 74% across 20 sites in the US for April 2020 compared to April 2019.
While people were encouraged to delay these essential screenings during the spring, we know that early detection of cancer is critical for achieving the best outcome and so we want to stress the importance of keeping up with your medical appointments and recommended screenings. To that end, we want to empower you with a set of questions to ask your doctor in advance of any visits so that you feel they are taking appropriate precautions to ensure your safety.
What Should I Ask My Doctor About What They’re Doing to Keep Me Safe?
It’s not unusual to be concerned about the risk of exposure to coronavirus when you go to a clinic or hospital during a pandemic. A facility that is currently experiencing a large volume of COVID-19 patients, or limiting certain procedures or services, may have limitations on which patients it can accommodate. However, most facilities are ready to welcome patients.
Hospital and clinic facilities are taking extra precautions to keep their patients safe. Many facilities are posting videos and information on their websites explaining which precautions they’ve implemented (here is an example video).
If you can’t find information online about the facility you want to visit, call the facility and ask about their precautions. Here are some questions you can ask your care provider or facility before an in-person appointment:
- Can the care provider conduct the visit via telemedicine? (This option requires a patient who doesn’t need an in-person consultation or procedure, AND who is comfortable with and has the equipment for conducting video meetings on a computer or smartphone).
- Can prescriptions be acquired through home delivery, mail order, or curbside pick-up?
- Does the facility require everyone to wear a face covering at all times?
- Does the facility direct patients who have COVID-19 to specific entrances or areas to minimize contact with other patients?
- Does the facility screen all staff for typical COVID-19 symptoms before they start their shifts?
- Does the facility have screeners at patient entrances to ask about known COVID-19 symptoms, take each visitor’s temperature, and ensure appropriate face coverings are worn (and provided, if necessary)?
- Does the facility limit nonessential companions for each patient to no more than a single individual who is free of known COVID-19 symptoms?
- Does the facility promote physical distancing through use of protective barriers, markers on the floor to indicate where to stand to stay 6 feet apart, and separating seats in waiting areas?
- Is each piece of equipment and appointment area cleaned between each use by a patient?
- Do enclosed treatment spaces (like MRI machines) have a waiting period between patients?
- Does the facility adhere to stringent and frequent cleaning protocols, especially in high-touch areas?
- Does the hospital allow visitors in patient rooms? If so, does it require them to check in at a nursing station or other screening area before entering patient’s room?
Additional steps YOU can take to help keep yourself safe before, during, and after a visit inside a hospital or clinic:
- Don a clean face covering before entering the facility, avoid touching it or your face during your time in the facility, and keep it on at all times unless a healthcare provider asks you to remove it.
- Wash your hands frequently. Bring hand sanitizer with you (just in case)
- Before meeting your healthcare provider, wash your hands or use hand sanitizer.
- When you get back to your car or your home, remove the mask carefully by touching only the ear loops. Use hand sanitizer after removing your mask.
- To be extra cautious, wash your hands and face covering and change your clothes when you get home. You might even take a shower. Wash the clothes you wore to the facility.
And lung cancer research continues in full swing!
This year’s World Conference on Lung Cancer (WCLC 2020), hosted by the International Association for the Study of Lung Cancer, went virtual due to the COVID-19 pandemic. Originally scheduled to be held in Singapore from August 8-12, 2020, the scientific sessions will be available from January 28-31, 2021.
WCLC 2020 was officially kicked off on August 8, 2020 with the Presidential Symposium live telecast at 7 PM Singapore time. The Presidential Symposium is a platform to present practice-changing research in the early detection or treatment of lung cancer. This year’s Symposium had three fantastic Phase III trial presentations on immunotherapy for non-small cell lung cancer (NSCLC), a new targeted therapy for ALK-positive lung cancer, and immunotherapy for mesothelioma.
- Currently, a chemotherapy -immunotherapy (pembrolizumab) combination is prescribed as first-line treatment for NSCLC that does not have any targetable driver mutations and that does not express high levels of PD-L1 protein. This is based on the results of the KEYNOTE-189 clinical trial, and the combination is available in the United States and some Western European countries. Results from the Phase III ORIENT-11 trial conducted in China show that addition of an immunotherapy (sintilimab – a PD-1 checkpoint inhibitor) to chemotherapy shows similar benefits seen in KEYNOTE-189. This is an extremely critical finding because results of the ORIENT trial will set the stage for this combination to be available in China and other Asian countries, so that patients can continue to benefit from these advances.
- Ensartinib is a 2nd-generation ALK tyrosine kinase inhibitor. Results from the Phase III eXalt3 trial comparing ensartinib to crizotinib as first-line treatment for ALK-positive lung cancer show that this 2nd generation ALK inhibitor is superior to crizotinib, in terms of its effect both on the primary lung cancer and on brain metastases. These exciting results suggest that ensartinib may be another treatment option for ALK-positive lung cancer in the first-line setting.
- Malignant pleural mesothelioma (MPM) is an aggressive type of cancer affecting the lining of the lungs. It has been associated with exposure to asbestos. Results from the phase III CheckMate 743 trial, comparing combination immunotherapy (nivolumab-ipililumab) to chemotherapy showed that immunotherapy combo is superior to chemotherapy, in the first-line setting.
These three presentations will likely set the foundation for new drug approvals and remind us that lung cancer research will continue, no matter what COVID-19 brings!
Resources and websites
- IASLC’s Guide to COVID-19 and Lung Cancer
- The National Cancer Institute has a special website for COVID-19 and emergency preparedness. COVID-19: What People with Cancer Should Know
- We are following updates provided by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC)
- Johns Hopkins COVID-19 Resource Center
- Interactive map of US COVID-19 cases by state
- The One-Two Punch: Cancer And COVID-19 (an important perspective for cancer patients)
- You can find information specific to your state or city or town on your health department’s website.
- American Medical Association resources for healthcare providers
GO2 Foundation for Lung Cancer (Amy Moore, PhD – amoore@go2foundation.org )
LUNGevity Foundation (Upal Basu Roy, PhD, MPH – ubasuroy@lungevity.org )
Lung Cancer Foundation of America (Kim Norris – KNorris@lcfamerica.org )
Lung Cancer Research Foundation (Cristina Chin, LMSW, MPH – cchin@lcrf.org)
LungCAN (Kimberly Lester – kimberly@lungcan.org)
July 27, 2020 Update
These updates began on March 3, 2020–a week before the World Health Organization declared COVID-19 a pandemic–when concerns arose in the lung cancer community regarding news out of China about a novel respiratory virus especially deadly to patients with lung cancer. Dr. Upal Basu Roy (who holds a Masters in Public Health), Dr. Amy Moore (whose PhD research was in virology), and Janet Freeman-Daily (a lung cancer research advocate) led lung cancer patient advocacy groups’ efforts to provide vetted, scientific information with a unified voice. Our goal is to provide a trusted source of information that each member of the community can use to assess their risk and make healthy choices for themselves and their families.
As of July 26, 2020, there have been over 16 million cases of COVID-19 worldwide. This week, the US surpassed 4 million cases– while our nation accounts for just over 4% of the world’s population, we make up 25% of virus cases. Another alarming statistic is the rapid pace with which we keep hitting stark new milestones – it took a mere 15 days for our cases to jump from 3 million to 4 million. These numbers reflect the exponential growth of viruses when appropriate public health measures are not heeded by enough members of the population.
SUMMARY OF AACR COVID-19 AND CANCER CONFERENCE
- The American Association of Cancer Research held a special virtual conference titled “COVID-19 and Cancer” on July 20-22, 2020. It is increasingly apparent that cancer and COVID-19 present a unique and unfortunate convergence, with lung cancer patients being among the most at risk for severe symptoms from the disease. This conference grew out of the research community’s need to understand the intersection of these two diseases and reflects the rapid mobilization of cancer scientists to apply their talents to finding solutions to this unprecedented global crisis. As one scientist stated, it is our “moral obligation” to help.
The lung cancer advocacy groups had two “poster” presentations at this conference. The first one summarized the origins of our joint COVID-19 statements and their impact on the lung cancer community. The second one discussed patient concerns that have emerged through these updates and how the advocacy groups can develop programs to address them.
- What is the latest data on risk of COVID-19 for lung cancer patients?
Several real-world studies were presented at the conference that addressed overall risk for cancer patients as well as lung cancer in particular. Real-world studies rely on data collected from patients receiving treatment at their regular cancer centers or hospitals (i.e. patients not receiving treatment through a clinical trial). Currently, real-world data seems to be the richest source of data for learning about how SARS-CoV-2 (as a virus) and COVID-19 (as a disease) impacts cancer patients.
Registry data is entered by the patient’s treating physician after the patient has a confirmed diagnosis of COVID-19. Data from two big registries were presented at this conference.
- The CCC19 registry is a multi-institutional, North American effort for all types of cancer. It reported that lung cancer patients were at higher risk of developing a more severe form of COVID-19. Other factors that predicted worse outcomes included older age, poor performance status, presence of co-morbidities, prior or current history of smoking, and a cancer that was progressing. The CCC-19 study showed an overall mortality of 26% for lung cancer patients with COVID-19, the highest of all the cancer types analyzed.
- The TERAVOLT registry is a multi-institutional, international effort dedicated to thoracic (lung-related) cancers. TERAVOLT data on 400 COVID-19 patients showed overall mortality of 35.5% for patients who had lung cancer and a higher mortality of 41% for patients who have SCLC. This increases the challenges presented by the pandemic to rural communities in the Southeast, where SCLC burden is high. Poor performance status was associated with more severe COVID-19 symptoms for SCLC patients. The patients in this study are primarily European, where the standard of cancer care may be different than in the US. It is important to keep in mind that SCLC is highly aggressive and has a higher symptom burden than NSCLC.
Single-institution data provide convenient samples to understand the natural history of a specific disease. At the conference, data from Memorial Sloan Kettering Cancer Center in New York City showed that prior immunotherapy for lung cancer did not impact outcomes of SARS-CoV-2 positive lung cancer patients. This data seems to contradict other registry-based efforts which have suggested that immunotherapy may predict worse outcomes. At the height of the pandemic in NYC, 20% of MSKCC’s lung cancer patients with COVID-19 died but many, including those with late-stage cancer, recovered. This study suggests patient-specific factors (such as type of treatment and patient characteristics) may determine overall risk and susceptibility to worse outcomes. It is important to keep in mind that standard of care and patient characteristics may be unique in a specific institution and therefore the results may not be generalizable.
One study presented at the conference that looked at electronic health records of patients in the US showed that an active cancer diagnosis coupled with co-morbidities such as diabetes and hypertension predicted worse outcomes for COVID-19.
Some common themes emerged for lung cancer patients:
- Patient-specific factors such as older age, presence of lung comorbidities such as COPD, and a poor performance status (higher than 1) are associated with a risk of developing a more severe form of COVID-19.
- Certain treatments such as chemotherapy (either alone or in combination) may increase the risk of developing a severe form of COVID-19 due to the immunosuppressive effects of chemotherapy.
We are still learning about how patient-specific factors and treatment-specific factors related to lung cancer can influence the severity of COVID-19. It is best to discuss how an individual patient’s situation will be impacted with the treating physician.
What is abundantly clear at this point is that multiple studies point to increased risk and worse outcomes in lung cancer patients with COVID-19. As the pandemic continues to spread throughout the US, it is imperative that lung cancer patients continue to take the threat seriously and take appropriate steps to protect themselves and those around them:
- limit unnecessary travel (particularly to areas where COVID-19 is prevalent),
- practice social distancing,
- wash hands frequently (or use hand sanitizers when handwashing is unavailable), and
- WEAR A MASK when out in public.
- How has the COVID-19 pandemic impacted oncologists and the cancer healthcare community?
The impact of the COVID-19 pandemic on the mental health of oncologists cannot be underestimated. Several studies suggest that oncologists will likely suffer from “burn-out” syndrome and post-traumatic stress disorder (PTSD). Two studies documenting the effect of the pandemic on mental health of oncology professionals were presented at the conference.
- One study looked at 300 oncologists in Western Europe and the United States during the first phase of the pandemic. Two biggest fears reported by the oncologists (almost 75% of participants) were “fear that their patients would get sick” and “fear that their family members would get sick.” Several oncologists opted to live away from their families during their oncology service to protect their families (Symposium 7, Dr. Gabriella Pravettoni).
- In the second study reported at the Keynote Symposium, which included 1570 oncologists from 102 countries, more than 75% of the oncologists reported that they feared contracting COVID-19 (July 21 Keynote, Dr. Solange Peters).
Both these studies highlight the importance of developing adequate mental health support services for healthcare professionals as the effects of the pandemic emerge.
As patients and advocates who work regularly and intimately with oncology healthcare professionals, we must not forget to express our gratitude to all members of the patient care team.
Resources and websites:
- IASLC’s Guide to COVID-19 and Lung Cancer
- The National Cancer Institute has a special website for COVID-19 and emergency preparedness. COVID-19: What People with Cancer Should Know
- We are following updates provided by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC)
- Johns Hopkins COVID-19 Resource Center
- Interactive map of US COVID-19 cases by state
- The One-Two Punch: Cancer And COVID-19 (an important perspective for cancer patients)
- You can find information specific to your state or city or town on your health department’s website.
- American Medical Association resources for healthcare providers.